1中国科学院上海药物研究所
2中科院
3赛诺菲公司,美国DMPK部门
药物相互作用
结果:
人OATP1B1/1B3摄取转运体(对应大鼠OATP1B2)将甘草酸从血液摄取进入肝脏,人外排转运体MRP2、BCRP、BSEP、MDR-1(对应大鼠MRP2/BCRP/BSEP)介导药物外排进入胆汁中。利福平(OATP1B抑制剂)将抑制大鼠肝脏摄取型转运体,导致甘草酸的系统暴露量明显增加;此外,甘草酸与血浆蛋白广泛结合,其肾小球滤过率较低。最终导致甘草酸体内暴露量较高。PBPK模型的定量分析表明当甘草酸与转运体抑制剂联合给药时,甘草酸药动学过程中发挥关键作用的OATP1B1/1B3将受到抑制,并引起潜在的药物相互作用DDI风险。
结论:
转运体介导甘草酸的肝脏摄取与胆汁排泄,影响其消除与药代动力学,定量分析OATP1B1/1B3转运体介导的甘草酸潜在DDI风险,可以增强甘草酸与其他药物合用治疗肝脏疾病的成功率。
KEY RESULTS
Hepatobiliary excretion of glycyrrhizin involved human OATP1B1/1B3-(orOatp1b2inrats)mediated hepatic uptake from blood and human multidrug resistance-associated protein (MRP)2/breast cancer resistance protein (BCRP)/bile salt export pump (BSEP)/multidrug resistance protein(MDR)1-(orMrp2/Bcrp/Bsepin rats)mediatedhepaticefflux into bile. Impairment of hepatic uptakein rats by rifampin resulted insignificantly increased systemic exposure to glycyrrhizin, which had slow glomerular-filtration-based renal excretion due to extensive protein-binding in plasma. Quantitative analysis using the PBPK model demonstrated the critical roles of OATP1B1/1B3 in pharmacokinetics ofglycyrrhizin,which hadhigh likelihoodto be avictimof drug-drug interactions when coadministered with potent dual inhibitors of these transporters.
CONCLUSION AND IMPLICATIONS
Transporter-mediated hepatobiliary excretion governs glycyrrhizin’selimination and pharmacokinetics. Understanding glycyrrhizin’s potential drug-drug interactions on OATP1B1/1B3 is expected to enhance success of glycyrrhizin-including combination drug therapies of liver diseases.
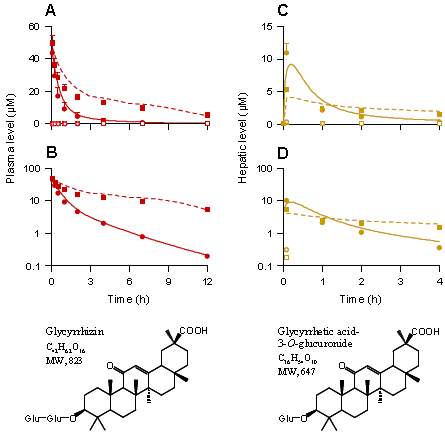
圆点代表不用利福平处理空白对照组大鼠,正方形点代表用利福平处理的大鼠,A和B图代表大鼠静脉注射2.6mg/kg 皂苷,血浆中甘草甜素和甘草酸-3-O-糖醛酸的浓度-时间曲线及对数图
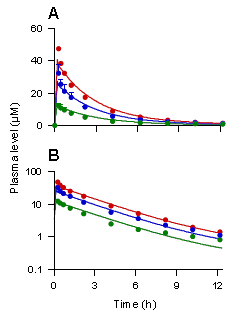
A和B图分别代表人静脉滴注甘草甜素40mg(绿色线)、80mg(蓝色线)、120mg(红色线)后,血浆中甘草甜素的浓度时间曲线图及对数图
下载该篇文章的英文原文献PDF文件: 002.Glycyrrhizin-has-a-high-likelihood-to-be-a-victim-of-drug-drug-interactions-mediated-by-hepatic-OATP1B1_1B3.pdf (下载800)
 协同生物
协同生物







您好!请登录